Testimony to FDA About Dendreon's Provenge, March 29, 2007
Dr. Ke Liu, MD, PhD
Medical Officer, Division of Clinical Evaluation,
Pharmacology and Toxicology,
CBER, FDA
DR. LIU: Good morning. My name is Ke Liu. I am the clinical reviewer for this BLA. And I'm going to present FDA clinical review and the findings efficacy and safety as outlined here.
Before I start, I'd like to make sure that all of us are on the same page in terms of terminology for my presentation. Study names Study 1 as sponsor referred to, D9901, and Study 2 meaning D9902A. So you see 1 is 1, 2 is 2. Study agents: sipuleucel-T you go to APC8015, and placebo meaning APC placebo, APC8015F meaning frozen and thawed peripheral blood mononuclear cells as source material, and then prepared similarly as sipuleucel-T.
Proposed indication for this BLA is for the treatment of men with asymptomatic metastatic androgen-independent prostate cancer, or AIPC. The efficacy - the basis for the efficacy claim is based on overall survival difference observed in two Phase III studies, D9901 and D9902A. In D9901, a 4.5-month overall survival difference was seen, and in D9902A, a 3.3-month overall survival was seen, but not statistically significant. These two Phase III studies were similarly designed, randomized, double blinded, placebo-controlled trials in men
with asymptomatic metastatic AIPC. The primary endpoint for each study was time-to disease-progression. D9901 enrolled 127 subjects, 82 in sipuleucel-T arm, 45 in placebo. D9902A planned 120 subject, but terminated early, as I will discuss later, contained 65 subjects in sipuleucel-T arm, 33 in placebo. Study periods are shown here. The key eligibility criteria, treatment schema and treatment regimen has been presented by the sponsor in detail. I will not discuss this further here.
Now I turn to study design. The primary endpoint for each study was time-to disease-progression as defined by time from randomization to the first observation of disease progression, and assessed by three criteria. First, radiologic progression by every eight weeks, CT or an MRI at baseline, and only if the results were positive, repeat every eight weeks. It should be noted that, by this study design, the soft tissue disease progression in bone-only subject may have been missed because of a lack of regular scans for soft tissue. The second criterion for the disease progression
was new onset of cancer-related pain correlated with X-ray findings. The third one was occurrence of the clinical events such as pathologic fracture, cord or nerve root compression, or other clinically significant disease-specific events. The second endpoint is shown on this slide. I am not going to read them.
Statistical assumptions are as follows. Based on sponsor's past Phase II experience and review of literature, the median time-to-progression was assumed for placebo arm to be 16 weeks. For the sipuleucel-T arm, predicted to be 31 weeks. The trial was designed with 2 to 1 randomization of sipuleucel-T to placebo, 80 percent power and 5 percent of two-sided alpha error.
Now I turn to efficacy results, starting with D9901 first, followed by D9902A. This slide shows D9901 patients' demographic and baseline characteristics. There's no significant imbalance between two arms for median age, ethnicity, or ECOG performance status. However, about 90 percent of subjects are Caucasian men, with 10 percent of subjects being other ethnic populations. Because of this under representation of other ethnic populations, it is not known whether the study results can be generalized to the general population, because the biology and prognosis of the prostate cancer in other ethnic populations may be different from those of Caucasian men. This slide shows distribution of disease status between the two arms in Study D9901 subjects. There are some imbalances noted in Gleason score, disease location, and number of bone metastases per subject. For example, sipuleucel-T arm had more subjects who had lower Gleason score, and more subjects with bone-only disease, and has more subjects with more than 10 bone metastases per subject than placebo. On the other hand, placebo arm had more subjects who had higher a Gleason score, and more subjects with disease lesions in both bone and soft tissue. These imbalances could have led to the biases to the study results. However, sensitivity analysis indicated that these imbalances did not have impact on overall survival results.
Now the results for D9901. Primary endpoint, time-to-disease progression, or TTP. One hundred twenty seven subjects randomized, 114 had disease progression events. No deaths prior to progression events. Progression was documented by imaging in 97 subjects, by clinical events in 10 subjects, and by new onset of disease-related pain correlated with imaging in seven subjects. Shown here is the Kaplan-Meier curves for primary endpoint TTP. 
FDA Graph.
Top curve sipuleucel-T, bottom curve APC placebo. Although the curve appears to be separating around Week 10, there was no overall statistical significance between the two curves. The p value was 0.085. Median TTP in sipuleucel-T arm was 11.1 week, placebo, 9.1 week. As you recall, the sponsor presented p-value of 0.052.
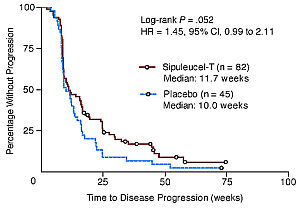
Dendreon graph. Click to enlarge
That was a change from 0.085 after initial analysis. This change from 0.085 to 0.052 was based upon unblended audit of clinical data, and revisions in the progression dates, primarily driven by the change of progression dates, or censoring from two subjects in a study with a small sample size.
In addition, difficulties in the interpretation of TTP results are shown in these slides. First, overestimation of time-to-progression. The sipuleucel-T arm presumed TTP was 31 weeks. Actually observed was only 11.1. That's about one third of the prediction, illustrating the overestimation of the TTP in sipuleucel-T based on non-randomized Phase II study. Second, median progression occurred before the scheduled second assessment for progression around Week 16. Third, lack of soft tissue scans in some bone-only subjects could have missed the detection of the soft tissue progression in the subject according to the study design. Lastly, some progression dates in some subjects were not interpretable because of the protocol violations. Thus, FDA considers the p-value of 0.05 by log rank test to be the primary results from the primary analysis specified in the protocol, and the p-value of 0.052 to be derived from an exploratory analysis. To conclude on TTP, D9901 failed to show a sipuleucel-T treatment effects on the primary endpoint in delaying time-to progression. There was no difference observed between the two arms for any of the following second endpoints as listed here.
Now, D9901 overall survival results.

FDA Graph
Shown here are the Kaplan-Meier survival curves for D9901 subjects. Top one is sipuleucel-T, bottom one is placebo. There was a separation of the curve occurring around Month 10, and this separation remains throughout the study period. There was an overall statistical significance between these two curves, p value equal to 0.10. Median survival time 10 for sipuleucel-T arm was 25.9 months, for placebo 21.4 months, 4.5-month difference. Looking at survival rate, at Month 36 where the data was cut off, 34 percent of sipuleucel-T subjects were still alive, and 11 percent of placebo subjects were still alive, 23 percent difference, also reached statistical significance. Dr. Bo-Guang Zhen will discuss to you about how to interpret those p-values in his presentation.
There are several factors that might have potentially compounded overall survival results observed in D9901. First was a crossover. This crossover could have actually negated the overall survival results observed in D9901. The other one is chemotherapy use. The higher percentage and earlier, longer, or higher dosage of chemotherapy in sipuleucel-T subjects could have led to increased overall survival difference observed in D9901. Now looking at crossover, 75.6 percent of placebo subjects was crossover to receive this APC8015F, a different product other than the sipuleucel-T. Looking at chemotherapy use, shown here is a percentage of the subjects who received chemotherapy after disease progression. Actually, the higher percentage of placebo subjects received chemotherapy, either taxane or any chemotherapy. Analysis of the time from randomization to first chemotherapy use also performed, which did not suggest an early initiation of chemotherapy in sipuleucel-T subjects. However, the dose and cycles of chemotherapy were not collected during study period. Thus, although unlikely, the potential chemotherapy confounding effects on overall survival cannot be ruled out.
To summarize for D9901 efficacy results, 127 subjects randomized 2 to 1, to sipuleucel-T, to placebo, a small sample size. No difference was observed between two arms in the pre-specified endpoint. Overall survival analysis, however, revealed a 4.5 months difference in the median survival in sipuleucel-T arm.
As Dr. Provost and Dr. Wonnacott described earlier, CD54 up-regulation was used in the potency measurement. Shown here is the correlation of the CD54 up-regulation and survival in Study D9901 subjects using the mean. The top curve is the curve for sipuleucel-T subjects whose CD54 up regulation above the mean, the middle curve is the subjects, sipuleucel-T subjects with CD54 up-regulation below the mean, and the
third curve is placebo subject. It appears that a higher CD54 up-regulation had better survival. However, the results are difficult to interpret because of the
following. It's not known whether this up regulation of CD54 results represents intrinsic property of the individual patients. Meaning, if patients are going to
do better would have a higher CD54 up regulation, or it's due to the intrinsic property of the individual products after manufacturing process. Should be noted that
the placebo cells did not undergo the similar manufacturing process as sipuleucel-T, or this up-regulation is due to other factors.
Another analysis, as Dr. Wonnacott alluded to earlier, was the T-cell stimulation immune response monitoring. Shown here are the T-cell stimulation assay in a limited number of sipuleucel-T and placebo subjects analyzed at Week 8 and Week 16, normalized to Week Zero, using antigens of PA2024 or human seminal PAP. End results are compared between the two arms. It appears that the sipuleucel-T subjects had a higher T-cell stimulation index. Again, the results are difficult to interpret because the proliferation assay used was not the direct measure for T-cell response, and assays performed were only in a small subset of patients. More difficult to interpret, as we had a little bit of discussion, was the fact there's no immune response were found to the human PAP
Now I turn to D9902A efficacy results. A little history about D9902. It was similarly designed as D9901, planned to enroll 120 subjects, and primary endpoint was time-to-disease-progression. It was terminated early because of D9901 overall negative efficacy results. At the time of termination, 98 subjects already enrolled. The study was renamed the D9902A.
of this early termination, this study contained insufficient sample size, not
powered to see a difference in TTP or overall survival. However, again noted is
90 percent of the study subjects being Caucasian men with under-representation of
other ethnic populations. This slide shows the distribution of disease status in D9902A subjects between the two arms. The same
16 patterns of imbalances were noted here in Gleason score, disease location, and number
18 of bony metastases per subject as noted in the Study D9901.
Now the results for D9902A. Primary endpoint time-to-disease progression. Shown here are two curves of sipuleucel-T and placebo Kaplan-Meier curves basically overlaps each other. No statistical significance. P-value is 0.719. The median time-to-progression was 10.9 weeks in sipuleucel-T arm, and 9.9 weeks in placebo arm, which was consistent with what's seen in Study D9901. Survival for D9902A. Shown here is the Kaplan-Meier survival curves. Top curve is sipuleucel-T, bottom curve is placebo. There was no overall statistical significance between these two curves. P-value equal to 0.331. Median survival time for sipuleucel-T, 19 months, and placebo, 15.7 months, 3.3 months difference. It should be noted that the survival time in this study was shorter than the counterparts in the D9901, which suggests that the patient populations in these two studies may not be exactly the same. To summarize for D9902A efficacy results, 98 subjects randomized 2 to 1 to sipuleucel-T to placebo. Similar trial design and execution as D9901. Stopped early, insufficient sample size to detect a difference in TTP or overall survival.
Now I turn to safety evaluation. The mean analysis were derived from D9901 and D9902A database, which included 146 subjects who received sipuleucel-T, and 76 subjects who received placebo. In addition, the sponsor submitted an updated information on cerebral vascular accident events, or CVA events, included CVA events from other Phase III trials, D9902B and P-11. The complete safety database update was suddenly last week to include a total of 461 subjects in sipuleucel-T, and 231 subjects who received a placebo. Looking at infusion exposure, vast majority of subjects received scheduled three infusions, about 90 percent in each arm. This slide shows death events in these two studies. Most subjects died from disease progression, and it appeared that fewer sipuleucel-T subjects died from prostate cancer, 65 percent versus 78 percent. No deaths were reported within 30 days after last infusion. Noted here was the deaths related to CVA increase in the sipuleucel-T arm, 4.6 percent versus 1.5 percent.
This slide shows serious adverse events other than death in these two studies. Noted again was the increased CVA events among other events in sipuleucel-T arm was 2.0 compared to none in placebo. This slide shows common adverse events that occurred in more than 10 percent sipuleucel-T subjects in these two studies. Adverse events listed here occurred more often in sipuleucel-T arms compared to placebo, including chills, pyrexia, headache, and others as listed in this table.
Now, I'll turn to the CVA events. As you saw previously, it appears that more CVA events were observed in sipuleucel-T subjects than in the placebo. The sponsor subsequently updated CVA safety information, which included D9902B, 198 subjects in sipuleucel-T, and 96 subjects in placebo. D9902B is another Phase III study with similar patient population as D9901 and D9902A. Ongoing, study is still blinded. Also updated information for CVA included subjects of sipuleucel-T, and 59 placebo. In another Phase III study, P-11, which closed to enrollment with a different patient population which was androgen dependent prostate cancer, gave rise to a total of subject number for the CVA summary of 461 for sipuleucel-T, and 231 for placebo.
For all subjects from these four randomized trials, the rate of CVA was 3.9 percent in sipuleucel-T compared to 0.6 percent in placebo, odds ratio 1.52. The deaths attributed to CVA was 1.5 percent in sipuleucel-T compared to 0.9 percent, odds ratio of 1.76. In the proposed indication for intended population, androgen independent prostate cancer, the CVA rate was 4.9 percent in sipuleucel-T compared to
1.7 percent in placebo. The deaths attributed to CVA in sipuleucel-T arm was 2.0 percent compared to 1.2 percent, the odds ratio 1.76. In P-11, the different patient population, ADPC, the CVA rate increase went to the other direction, higher in the placebo arm. Percentage was 5.1 percent compared to 0.9 percent in sipuleucel-T. And no deaths were attributable to CVA in P-11. So overall in these four Phase III trials, a higher percentage of CVA event was observed in subjects who received sipuleucel-T, 1.3 percent more than the placebo.
To conclude on safety, almost all sipuleucel-T subjects developed adverse events, not different from placebo. Most AEs were Grade I or II, and resolved within 48 hours. Twenty-four percent sipuleucel-T subjects developed serious adverse events not different from 23 percent of placebo treated subjects. Although the difference did not reach statistical significance, the increased CVA events observed in sipuleucel-T subjects is a potential safety signal.
To conclude on efficacy, neither studies of D9901 and D9902A met prespecified efficacy endpoint. However, survival analysis revealed a 4.5-month overall survival difference, statistically significant in D9901, and a 3.3-month overall survival difference in D9902A, which was not statistically significant. This slide shows the advantage of using overall survival in cancer clinical trials as contained in the FDA draft guidance document entitled Clinical Trial Endpoints for the Approval of Cancer Drugs in Biologics.

Overall survival is the most reliable cancer endpoint, usually the preferred endpoint, and studies can be conducted to adequately assess it. An improvement in survival is a clinical benefit. The endpoint is precise and easy to measure, document by the date of death. Bias is not a factor in endpoint measurement. Demonstration of a statistical significant improvement in overall survival has supported new drug approvals.
Now, let's look at overall survival difference in D9901. This 4.5-month median survival difference is clinically meaningful, but it has the following limitations, as Dr. Bo-Guang Zhen will discuss in detail in his presentation. First, post hoc analysis. All survival analysis were done post hoc, because survival was not the pre-specified endpoint, the primary method for survival analysis, and its comparison was not pre-specified. Second, it's one study with a small sample size, so the difference could be due to chance alone. Therefore, uncertainties exist regarding the persuasiveness of the
survival results in the support of sipuleucel-T BLA efficacy claim, and that's the reason why we're all here to discuss these issues today, and FDA would like to seek advice from the advisory committee. Now I turn the podium to Dr. Bo-Guang Zhen, who is going to discuss the overall survival difference from statistical perspective.
DR. MULÃ: Thanks, Dr. Liu.
More like this:
Testimony from Prostate Cancer
Patients and Partners,
FDA, March 29 2007.
Complete Record of FDA Meeting, .pdf
Complete Slides for Dr. Lui's presentation
FDA Clinical Review and Findings, Ke Lie, MD, PhD ( ppt ) ( htm )
News reports and releases and about Provenge
and other vaccines