Antibiotic Research May Yield Cancer Breakthrough
Scientists have made a discovery about antibiotics that may advance cancer therapy. By studying the mechanisms at work in protein production, a Princeton-led team has discovered why certain kinds of antibiotics are so effective.
The new discovery exposes how a specific protein protects against cell death. This may also shed light on the cancer-fighting process, because cancer involves inability of defective cells to die off.
In particular, the new discovery is relevant to study of a human protein known as Bax Inhibitor-1 (BI-1) that has been found to affect metastatic spread of prostate and other cancers.
In a study appearing in the Aug. 7 edition of the journal Science, Thomas Silhavy, Princeton’s Warner-Lambert Parke-Davis Professor of Molecular Biology, and Johna van Stelten, a graduate student, working with two Swiss researchers have uncovered how two antibiotics in common use for 50 years — tetracycline and chloramphenicol — can be supremely lethal against certain strains of bacteria.
Simply put, the researchers say, these drugs plug things up. If aimed at cancer cells in the human body, they theorize, this feature of the drugs’ effects might fight the disease.
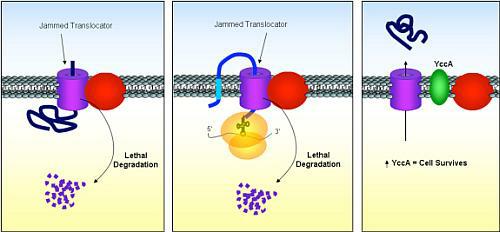
Silhavy and van Stelten had been studying how proteins — from antibodies to hormones — are produced in bacteria’s cytoplasm, the jelly-like material that fills cells, and how the proteins are then transported where they are needed. The spaghetti-like proteins exit the bacteria’s cytoplasm through microscopic tubes known as translocators (see diagram below).
Sometimes, proteins fold up accidentally and jam the translocator. “Proteins go through the translocator, like a piece of spaghetti through a hole,” Silhavy said. “But if you can imagine if you were to tie knots in the spaghetti, it wouldn’t be able to get through; it gets stuck.”
What happens then, according to Silhavy and van Stelten, who were the first ever to observe the event, is that the bacterial cell actually attacks the jammed translocator, decimating it.
New cell machinery unveiled:
(1) A Princeton-led team has discovered new mechanisms at work in protein production. Here, a folded protein (the ribbon-like object) jams a translocator (purple cylinder on left). Once jammed, translocators emit a molecular signal that attracts a destructive enzyme, the protease FtsH (red sphere), which then starts shredding the translocator (chopped up bits at bottom). (2) Similarly, when certain antibiotics are added to a bacterium’s cytoplasm, the ribosome — the cell’s protein-producing machine (yellow area) — stops midway through its process, causing partially constructed proteins to stick to the ribosome and jam the translocator. (3) When scientists increase the amount of YccA (green ellipse), a protective protein, in the cell, the protein protects the translocator from the FtsH attacker. (Image: Courtesy of Silhavy Laboratory)
The scientists wondered what might happen if antibiotics were introduced into the cell cytoplasm to purposely thwart bacteria.
They found that tetracycline and chloramphenicol cause the ribosomes, a cell’s protein-producing machines, to stop midway through the process of making proteins, leaving partially constructed proteins stuck to the ribosome, jamming the translocator in the bacteria.
“This is very similar to plugging the translocator with a folded protein and, sure enough, this also causes translocator destruction,” Silhavy said. “It’s like putting an anchor on the spaghetti instead of a knot. They are stuck and dead forever.”
When the translocators in bacteria become jammed like this, the researchers observed that they emit a molecular signal — a stress response — that calls in a destructive enzyme known as the FtsH protease. Under normal circumstances, the FtsH protease chops up the jammed translocators, contributing to cell death.
The scientists found, however, that when they increased the amount of a protein called YccA, present in the bacterial cell, YccA protected the translocators from the FtsH attackers. YccA, it turns out, is very similar to a human protein known as Bax Inhibitor-1 (BI-1) that is of great interest to cancer researchers because when it malfunctions, cancer spreads.
“We have determined how YccA works in preventing stress-induced death in bacteria,” van Stelten said. “We hope this new information will shed light on the mechanism of BI-1 in humans.”
Other researchers on the paper included Filo Silva and Dominique Belin from the University of Geneva in Switzerland.
The work was supported by the National Institute of General Medical Sciences of the National Institutes of Health, the New Jersey Commission on Cancer Research, the Canton de Geneve and the Swiss National Science Foundation.
Source — this article, written by Kitta MacPherson for Princeton University News, has been edited for psa-rising.com by J. Strax.
Related
Effects of Antibiotics and a Proto-Oncogene Homolog on Destruction of Protein Translocator SecY Science August 7 2009

Comments are closed.